“The important thing in science is not so much to obtain new facts as to discover new ways of thinking about them.”― Sir William Bragg
From the week 9 article readings a common thread is the sharing of information to gain knowledge and improve the care of patients, through open data bases, artificial intelligence, and sharing of research information. For anyone who has worked or followed the continuing advancements in oncology care, it is easy to see how the open sharing of knowledge between providers and researchers could significantly impact care. Oncology care continues to rapidly change as new knowledge is learned – from increased understanding at the molecular level, novel therapies, targeted therapies, and harnessing the immune system to fight cancer (DeVito, Leavitt, & Saleh, 2019).
Karen Olsen, PhD interviewed oncology experts regarding what to expect in 2020. One expert discussed continued study and advancements in the understanding and utilization of the immune system to treat cancer is predicted – immunotherapy. Additionally, another expert described the focus of individualized medicine or precision medicine where treatments are modeled after the specific pathology, tumor, and the specifics of the cancer identified with the help of technology.
Sounds AMAZING, right? But how do we get there? Knowledge. Clinicians and researchers need to share knowledge, as proposed as the ability to create a large data base where data can be compiled but to be successful requires identification of the best way with regulatory support in this data sharing (Olsen, 2020).
There are hundreds of clinical trials being completed all over the globe to advance our knowledge of cancer biology and treatments, imagine if collaboration was fostered. The Oncology Research Information Exchange Network (ORIEN) has sought to do just that. There are 19 cancer centers in the US listed as members of ORIEN. Described as a team environment of collaboration with the ability to use the strength of larger data sets to impact cancer care. Patients consent to share their clinical and molecular data with ORIEN.
For women with breast cancer, though a common disease with approximately 325,000 new cases of breast cancer (invasive and in-situ) estimated for 2020 by ACS, additional research is needed to better understand the potential survival benefit of prophylactic mastectomy, the reasons women chose to undergo a preventative mastectomy, and any potential cost savings to both the patient and the health care system. Specifically, in relation to prophylactic mastectomy benefits for medicare beneficiaries. A private sector collaborative entity like ORIEN would allow for sharing of data and findings of women across the US as opposed to the longer process of conducting multiple clinical trials. Thus potentially positively impacting the ability to affect legislation for the care of breast cancer patients in less time.
References:
American Cancer Society (2020). About breast cancer. [webpage]. Retrieved from: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
DeVito, T., Leavitt, J., & Saleh, N. (2019, January 17). Key advances in oncology, 2018. Oncology Journal, 33(1), 6-8. Retrieved from: https://www.cancernetwork.com/oncology-journal/key-advances-oncology-2018
LibQuotes (2020). William Lawrence Bragg quotes [webpage]. Retrieved from: https://libquotes.com/william-lawrence-bragg
M2Gen (2020). Oncolocy Research Information Exchange Network [webpage]. Retrieved from: https://www.oriencancer.org/
Olsen, K. (2020, January 10). Experts forecast cancer research and treatment advances for 2020. American Association for Cancer Research [webpage]. Retrieved from: https://www.aacr.org/professionals/blog/experts-forecast-cancer-research-and-treatment-advances-in-2020/
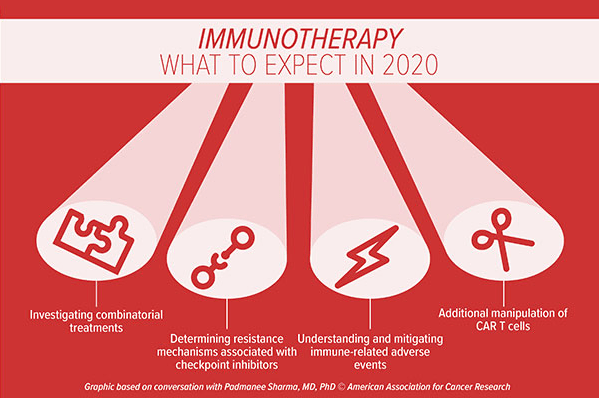
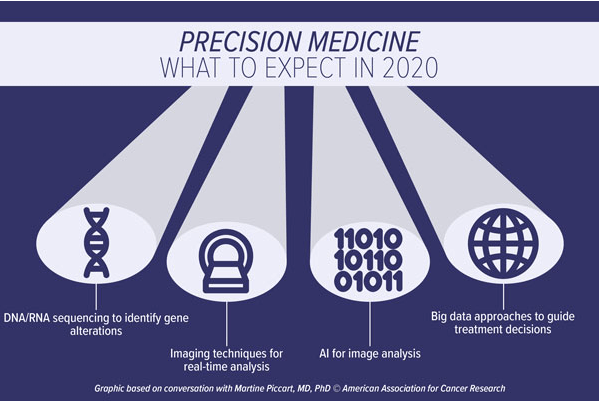
Data sharing is such an excellent idea. Imagine how advanced medicine can become in a short amount of time. Sharing information to gain knowledge, learn new perspectives, and enhance patient healthcare access and care sounds superb. The practice of making dating is about having scholarly research available to other researchers in the field of interests. Many funded agencies, institutions, and publication venues require authors of peer-reviewed-papers to share raw data and statistical methods. This helps the researchers understand, develop, and reproduce the published research.
However, some agencies limit or prohibit data sharing in order to protect proprietary interests, national security, subject confidentiality, and protection from use of data for political purposes. Data sharing and archiving is the long-term storage of scholarly data. The purpose is to allow other researchers to replicate the study and to test the research. However, this approach has been concerning due to the fact that some research heavily depend on the large data sets which cannot be easily replicated. For example, in drug development it is important to generate data that can verify the reports and drug companies publish accurate information. Therefore, it is key that a consensual agreement on the criteria/requirements have mutual recognition for the respective contributions.
It is unfortunate that data withholding can happen or authors refuse to provide data. If that is the case, then authors risk losing trust from the scientific community.
How applicable is this concept in regards to the COVID crisis? Do you know about the data sharing laws?
I look forward to our discussion.
Reference
United States. Congress. Office of Technology Assessment. (1987). Defending secrets, sharing data : New locks and keys for electronic information. Retrieved from https://congressional-proquest-com.ezproxy1.lib.asu.edu/congressional/result/pqpresultpage.gispdfhitspanel.pdflink/$2fapp-bin$2fgis-congresearch$2fe$2f8$2ff$2f8$2fcmp-1987-ota-0088_0001_from_1_to_50.pdf/entitlementkeys=1234%7Capp-gis%7Ccongresearch%7Ccmp-1987-ota-0088
LikeLike
Mae,
No, I am not overly familiar with the laws of data locks but of course have heard about intellectual property laws where trademarks and copyrights come from. Overall, I feel that in the oncology practice there is little “hoarding” of data. The treatment plans that we use are based upon clinical trial data. The recipe book of oncology care – the NCCN guidelines – are used universally in oncology and is developed through review of current research and best practices. In my experience, oncology care is where clinicians readily share information and collaborate to improve the care of their patients. I have seen instances where a patient has a rare form of cancer and the treating oncologist will contact the leading specialist in that disease to collaborate on the case. We have weekly tumor boards where a room full of oncology providers discuss cases.
When thinking about sharing data and laws outside of oncology, and the current COVID19 pandemic – reportable diseases to the CDC comes to mind. The CDC (2018) identifies that there are mandatory reportable diseases that are reported to the State Health Department, which is then reported to the CDC, however the reportable illnesses are shared voluntarily by the State Health Departments with the CDC. This data is collected in a large system (NNDSS) that is coordinated at the national level to review and analyze data and distribution of the notifiable disease data. I anticipate that COVID19 will be reported like influenza moving forward.
The challenges discussed in big data, is keeping the data safe and not misusing the data. We have all seen and heard about data breaches where anything from medical records to social security numbers have been leaked. Thus, when looking at data collection – I think it is important to collect data without patient identifiers, much like we do in research. Another challenge is consent, participants must consent to sharing their health information. In my current role as a research nurse, patients are notified that the specimens (blood, tumor, CSF, etc.) will be kept by the company and may be utilized in the future for, yet unknown, research. In my experience patients are altruistic, hoping that what they donate will help discover novel treatments or improve current care.
Do you think that large data sets should be mandated by private sector, or public sector, or a partnership? How to we obtain consent to cover the use of data when the full extent of use may not be known?
Reference:
Centers for Disease Control and Prevention (2018). National Notifiable Diseases Surveillance System {webpage]. Retrieved from: https://wwwn.cdc.gov/nndss/data-collection.html
LikeLike